|
<NOTE>
Published online (June 25, 2020)
Cultural dietary stasis? Four decades on, Mahale chimpanzees still favour Macrotermes
Alejandra Pascual-Garrido1
1 Primate Models for Behavioural Evolution Lab, Institute of Cognitive and Evolutionary Anthropology, School of Anthropology and Museum Ethnography, University of Oxford, 64 Banbury Road, Oxford OX2 6 PN, United Kingdom 2 Fort Lauderdale Research and Education Center, University of Florida, 3205 College Avenue, Davie, FL 33314, USA INTRODUCTION Termite consumption by wild chimpanzees (Pan troglodytes) is widespread across Africa (McGrew 1992) but not universal (Fowler & Sommer 2007). When consumed, termites represent a valuable source of energy, fat, high-quality protein, minerals and vitamins (O’Malley & Power 2014). Frequency, acquisition techniques and choice of prey vary greatly among populations (Wondra et al. 2016). While some of these differences respond to the characteristics of the termite prey and ecological context, other aspects may more easily be attributed to social influences (Sanz et al. 2014). Of the 106 genera of soil-dwelling termites present in Afrotropical habitats (Krishna et al. 2013), only eight have been recorded as chimpanzee termite prey, with Macrotermes being the genus mostly eaten (Lesnik 2014). Chimpanzees of Bilenge (B group) inhabiting the Mahale Mountains National Park in Tanzania were first reported to harvest Macrotermes with tools in 1975 (Nishida & Uehara 1980). Other termites such as Odontotermes and Pseudacanthotermes spiniger were more abundant but ignored (Collins & McGrew 1985). Recent studies revealed that the B group has maintained their termite-fishing tradition for more than forty years (Pascual-Garrido 2017). However, if they continue to favour Macrotermes remains unknown. Here we document termite prey consumption by Mahale B group chimpanzees and compare with those reported decades ago (Collins & McGrew 1985). We discuss possible factors influencing this choice. 


Figure 1. Termite genera in Bilenge. a) APG and a mound of Pseudacanthotermes spiniger; b) Odontotermes; c) Macrotermes michaelseni with recently abandoned termite fishing tools by chimpanzees (01 Dec 2014). Photo credits: Alejandra Pascual-Garrido. 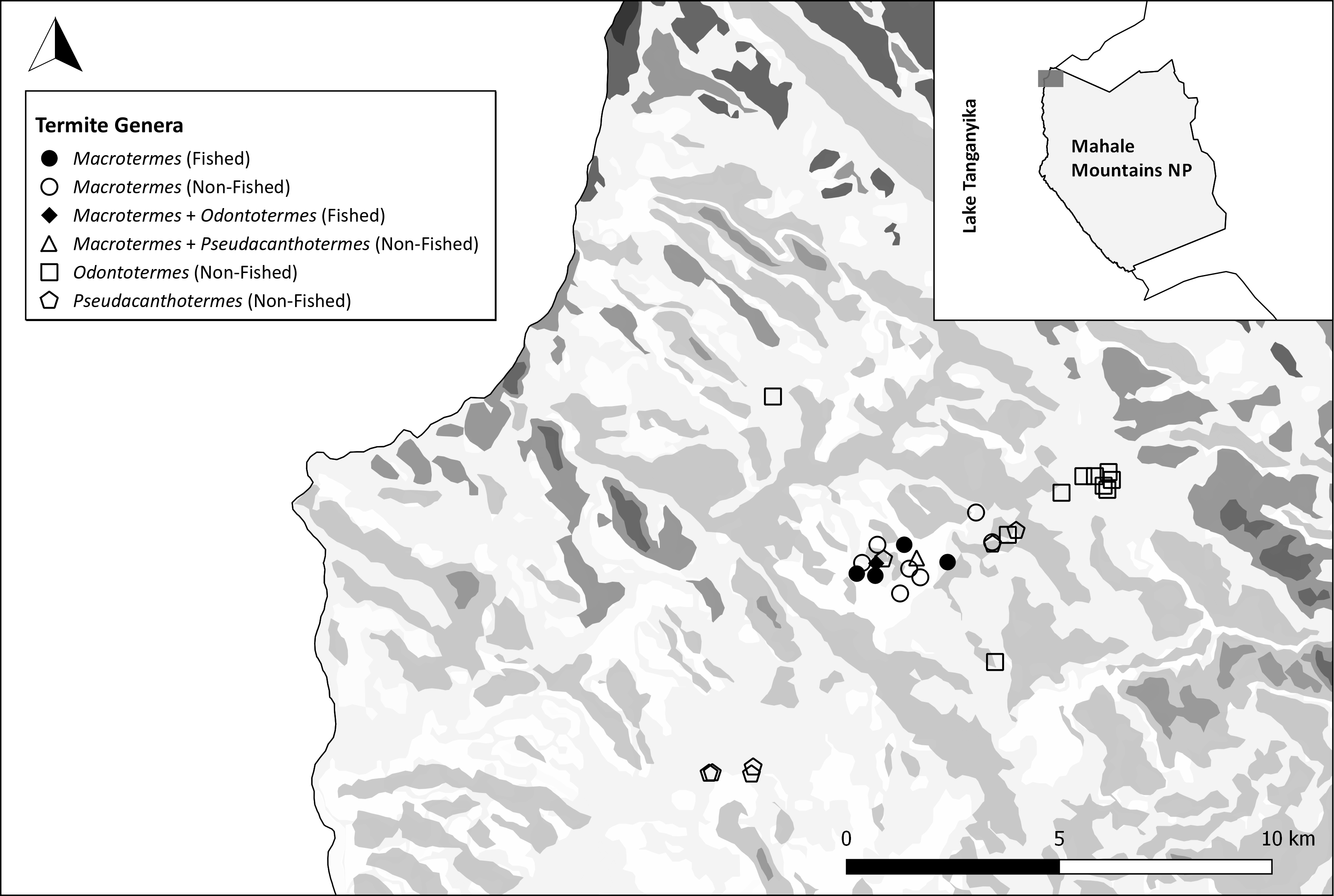
Figure 2. Map of the distribution of termite mounds surveyed at Bilenge. Mounds fished by chimpanzees are shown in black. Map credits: Katarina Almeida-Warren. Table 1. Termite family, subfamily, genera and species surveyed at Bilenge. Mounds targeted by chimpanzees appear in grey. 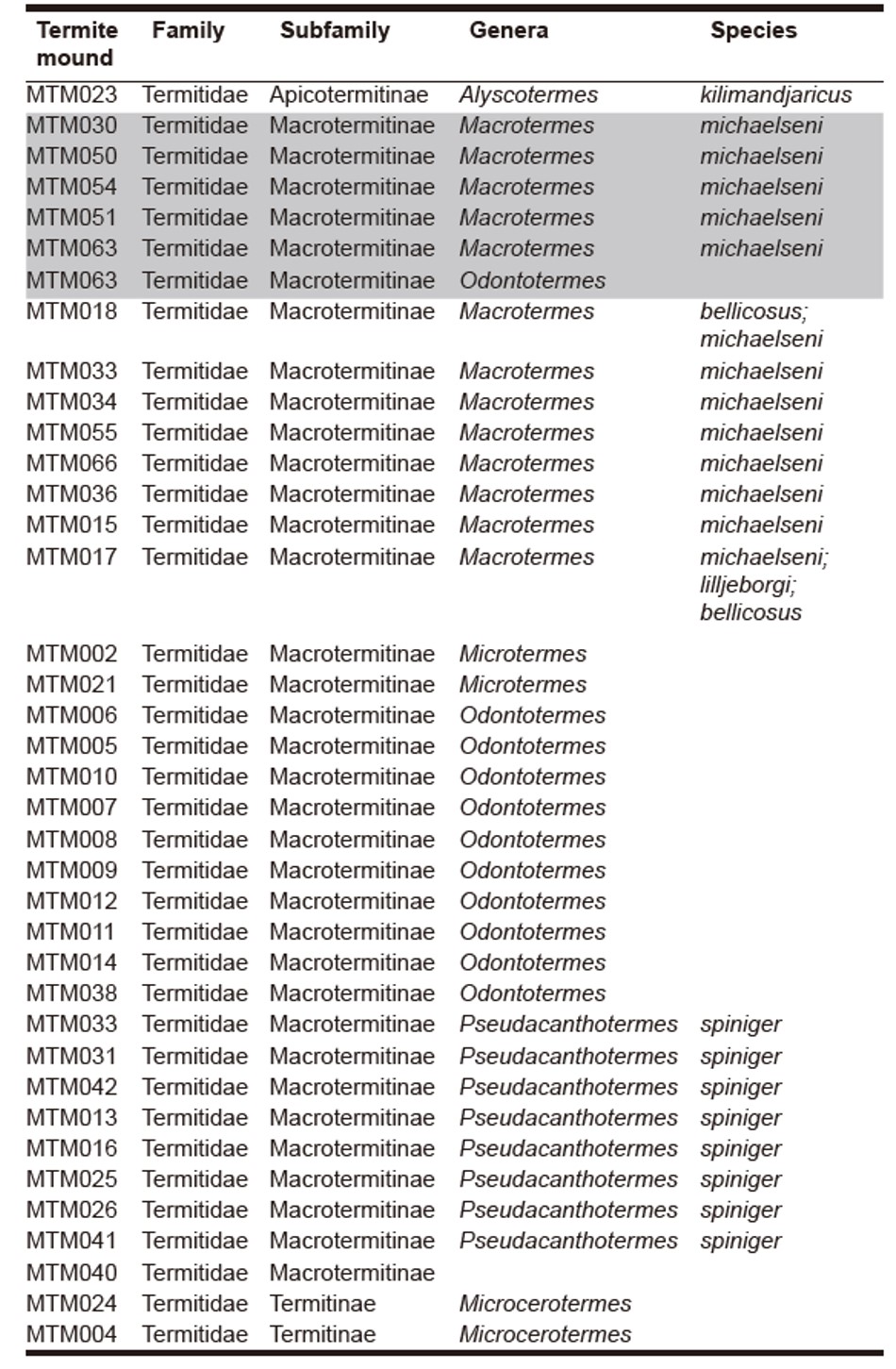
METHODS The unhabituated chimpanzees (Pan troglodytes schweinfurthii) of B group live in Bilenge (6° 2' S, 29° 44' E; 772–1550 m elevation), the northwestern edge of the Mahale Mountains National Park in western Tanzania (Nishida & Uehara 1980). The dry season from mid-May to mid-October has virtually no rain. The rainy season generally has two peaks of rainfall in November and in March-April, with an average annual rainfall of about 1400 mm. Bilenge is mostly dominated by open grassy woodland, mainly Brachystegia (miombo), with vine tangles and forests only present in narrow strips along the valleys, and broad hilltops rising from the coastal plain (Collins & McGrew 1988). Termite-fishing though habitual, is highly seasonal, mainly taking place during the first half of the wet season (from October until at least February), which coincides with the annual and dispersal flights of the termites (Uehara 1982). Three field seasons were conducted by APG assisted by one experienced Tanzanian field assistant (25 Nov–14 Dec 2014; 20 Oct–08 Nov 2015; 21 Nov–08 Dec 2016). During reconnaissance surveys, APG searched for mounds which could potentially have been fished by chimpanzees within B’s group range (Nishida & Uehara 1980). Mounds included broadly domed mounds, mounds with open ventilation holes and towers raised as chimney (Collins & McGrew 1985). Smaller mushroom-shaped mounds, typical of soil Cubitermes, were ignored given that they are not targeted with plant tools by chimpanzees (Collins & McGrew 1985; O’Malley & Power 2012) (though Bili-Uére chimpanzees use percussive technology to access them; Hicks et al. 2020). Data collected at each mound included GPS position and, based on archaeological methods (Pascual-Garrido 2018), evidence of each mound having been targeted by chimpanzees including: (a) tools, (b) plants sourced for tool material, (c) signs of damage (i.e. knuckleprints). For each mound, at least 10 termite specimens were collected, fixed in ethanol 80% and later identified by RS. Specimens collected included mostly soldiers. Workers and alates were collected when available. RESULTS A total of 36 mounds were sampled and identified. This included six genera and five species, with Macrotermes, Pseudacanthotermes and Odontotermes being the genera most represented (Figure 1). All mounds were occupied by a single resident species, except for four mounds where multiple occupants resided (Table 1). Soldiers were collected at all mounds, with alates only present at three Macrotermes mounds and at one Odontotermes mound. Only Macrotermes michaelseni mounds showed signs of having been targeted by chimpanzees (n=5), one or multiple times (Pascual-Garrido 2017), and one also occupied with a second resident from the genus Odontotermes (Table 1; Figure 2). No evidence of chimpanzee predation was recorded for any other termite genera. Evidence of chimpanzee predation at mounds included tools (n=1), knuckleprints (n=1) and plants sourced for tool materials (n=5) (Pascual-Garrido 2017). DISCUSSION More than 40 years have passed and successive generations of Bilenge chimpanzees continue to fish Macrotermes mounds while ignoring other termites also common in the area (Collins & McGrew 1985). Macrotermes are preyed on by more populations of chimpanzees than all other termite taxa, being the best-documented insect genus for ape use of elementary technology in extractive foraging (Lonsdorf 2005). Given the high nutritional value, fishability, greater mass and less noxious taste of these fungus-farming termites compared to other genera (Collins & McGrew 1985; O’Malley & Power 2014), it is therefore not surprising that Bilenge chimpanzees continue to fish them. But why do Bilenge chimpanzees still ignore other genera such as Odontotermes and Pseudacanthotermes? The absence of Odontotermes’ consumption, a genus not consumed by any wild chimpanzee population (except rehabilitated chimpanzees released in Rubondo Island in Tanzania; Moscovice et al. 2007), is not surprising: their distasteful defensive secretion makes them unpalatable for chimpanzees – and for humans too as corroborated by APG. More puzzling is the lack of ingestion of the highly nutritional and more abundant P. spiniger – a species habitually fished by the extinct neighbouring K group of Mahale (Uehara 1982) and by Issa chimpanzees living 114 km east of Bilenge (Stewart & Piel 2014; Pascual-Garrido, unpublished data). It may be that Bilenge apes fish P. spiniger later in the wet season (although Uehara’s (1982) analysis from faecal samples suggests that they do not). That we failed to find any evidence of termite-fishing at these mounds, including plants used for tool making which, unlike tools, remain detectable for years (Pascual-Garrido 2018), makes this unlikely. If members of the B group eat alates (and soil) of P. spiniger, as neighbouring (extinct) K group and M group do without tools (Uehara 1982) (though see Takahata 1982 for use of tools by two immigrant chimpanzees from K group termite fishing at P. spiniger mounds), we would have missed it, as this can only be evidenced by direct observation of the apes (not possible at Bilenge) or by faecal samples inspection (not included in this study). Still, at present we have no evidence for this – and certainly no signs at all that they fish them. The question therefore remains: Why do Bilenge chimpanzees focus their termite-fishing exclusively on Macrotermes, as do Gombe chimpanzees, while ignoring P. spiniger whose major soldiers are high in energy, fat and protein and fished by other chimpanzee communities (Uehara 1982; O’Malley & Power 2012)? One possibility could be that the density of Pseudacanthotermes at Bilenge has declined during the last decades and chimpanzees are selecting their prey subject to their availability. However, earlier studies indicate this is not the case (Collins & McGrew 1985), but that P. spiniger are ignored because of the soldier’s smaller size and less value on a per-foraging-unit basis compared to Macrotermes (O’Malley & Power 2012). However, an extended termite fishing session on P. spiniger could still provide a nutritional meaningful yield of energy, fat and protein (O’Malley & Power 2012). Previous research indicates that techniques and type of tools used to harvest insects, including termites, is partly influenced by the behaviour of the species preyed upon (Schöning et al. 2008; Sanz et al. 2014). If Macrotermes are more fishable than Pseudacanthotermes (i.e. grip on the tool with greater force) remains to be tested. Another possibility, perhaps more difficult to test, is that chimpanzees may be reluctant to try new food choices. Adult chimpanzees are conservative regarding their feeding habits, which can act as a selective force against a new food (Nishida et al. 1983). Still, they may consume a variety of human (crop) foods if available (McLennan & Hockings 2014). Furthermore, captive and wild chimpanzees show a marked persistence for initially adopted strategies related to the acquisition of food, even when a strategy stops being successful (Gruber et al. 2009; Hrubesch et al. 2009). Conservatism can create within-group behavioural homogeneity, while Whiten et al. (2005) attributed it to conformity. Chimpanzees are highly selective regarding their insect prey choices (Webster et al. 2014). That some chimpanzee communities prey on termites, while others do not (Fowler & Sommer 2007), and that harvesting techniques differ between (and within) communities (McGrew 1983; Boesch et al. 2020), highlights the importance of investigating the mechanisms behind these differences. Social influences on food preferences are barely contemplated in current studies of chimpanzee culture (but see Hicks et al. 2020) – maybe because variation in feeding behaviour is more parsimoniously explained by ecological differences (Byrne 2007). However, Boesch et al. (2006) suggested that variation in feeding time relative to fruit availability may reflect cultural differences, while Hicks et al. (2020) found no association between insect availability and consumption. Likewise, Nishida et al. (1983) and Sakamaki et al. (2007) reported that two neighbouring groups at Mahale consume different food items, despite their equal availability in both areas. Similarly, neighbouring chimpanzee communities living in the Budongo Forest Reserve, Uganda, vary in their preference for red duiker (Cephalophus natalensis) consumption despite no difference in prey distribution (Hobaiter et al. 2017). Social learning plays a fundamental role in the acquisition of technological skills to harvest social insects, including food choices (Lonsdorf 2005; Schöning et al. 2008; O’Malley et al. 2012), and is key for the development and maintenance of cultural behaviours and traditions in primates (Schuppli & van Schaik 2019). Further research is warranted to establish if social learning in chimpanzees extends to their choice of termite prey. ACKNOWLEDGMENTS We thank field assistants Shaka and Singa Kabugonga for their invaluable help and guidance in the field. Special thanks go to Dr Michio Nakamura for aiding logistics and providing advice in the field. We thank Edward Kohi, Pius Kavana and Maria Mbogo for safekeeping APG’s equipment in Kigoma town. Research permission to conduct research in Mahale Mountains National Park was granted by Tanzania National Parks (TANAPA), Tanzania Wildlife Research Institute (TAWIRI) and Tanzania Commission for Science and Technology (COSTECH). Logistical support was provided by Mahale Mountains Chimpanzee Research Project. We are very grateful to Katarina Almeida-Warren for production of the map. We thank William McGrew for very helpful comments on an earlier version of this manuscript. We want to dedicate this work to the late Prof. Shigeo Uehara whose pioneering work at Mahale made a vital contribution to our understanding of chimpanzees and termites. APG was supported by a Leverhulme Trust Early Career Fellowship and received further funding for fieldwork from The Boise Trust Fund, University of Oxford. REFERENCES Boesch C, Gone Bi ZB, Anderson DP, Stahl D 2006. Food choice in Taï chimpanzees: Are cultural differences present? In: Feeding Ecology in Apes and other Primates: Ecological, Physical and Behavioral Aspects. Hohmann G, Robbins MM, Boesch C (eds), Cambridge University Press, New York, pp 183–201. Boesch C, Kalan AK, Mundry R et al. 2020. Chimpanzee ethnography reveals unexpected cultural diversity. Nat. Hum. Behav. https://doi.org/10.1038/s41562-020-0890-1 Byrne RW 2007. Culture in great apes: Using intricate complexity in feeding skills to trace the evolutionary origin of human technological prowess. Philos Trans R Soc London B Biol Sci 362:577–585. https://doi.org/10.1098/rstb.2006.1996 Collins DA, McGrew WC 1985. Chimpanzees’ (Pan troglodytes) choice of prey among termites (Macrotermitinae) in western Tanzania. Primates 26:375–389. https://doi.org/10.1007/BF02382454 Collins DA, McGrew WC 1988. Habitats of three groups of chimpanzees (Pan troglodytes) in western Tanzania compared. J Hum Evol 17:553–574. https://doi.org/10.1016/0047-2484(88)90084-X Fowler A, Sommer V 2007. Subsistence technology of Nigerian chimpanzees. Int J Primatol 28:997–1023. https://doi.org/10.1007/s10764-007-9166-0 Gruber T, Muller MN, Strimling P, Wrangham R, Zuberbühler K 2009. Wild chimpanzees rely on cultural knowledge to solve an experimental honey acquisition task. Curr Biol 19:1806–1810. https://doi.org/10.1016/j.cub.2009.08.060 Hicks TC, Kühl HS, Boesch C et al. 2020. The relationship between tool use and prey availability in chimpanzees (Pan troglodytes schweinfurthii) of Northern Democratic Republic of Congo. Int J Primatol. https://doi.org/10.1007/s10764-020-00149-4 Hobaiter C, Samuni L, Mullins C, Akankwasa WJ, Zuberbühler K 2017. Variation in hunting behaviour in neighbouring chimpanzee communities in the Budongo forest, Uganda. PLoS ONE 12:e0178065. https://doi.org/10.1371/journal.pone.0178065 Hrubesch C, Preuschoft S, van Schaik C 2009. Skill mastery inhibits adoption of observed alternative solutions among chimpanzees (Pan troglodytes). Anim Cogn 12:209–216. https://doi.org/10.1007/s10071-008-0183-y Krishna K, Grimaldi DA, Krishna V, Engel MS 2013. Treatise on the Isoptera of the world 1. Introduction. Amnh Bulletin 377:1–200. https://doi.org/10.1206/377.1 Lesnik JJ 2014. Termites in the hominin diet: A meta-analysis of termite genera, species and caste as a dietary supplement for South African robust australopithecines. J Hum Evol 71:94–104. https://doi.org/ 10.1016/j.jhevol.2013.07.015 Lonsdorf EV 2005. Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim Behav 70:673–683. https://doi.org/10.1016/j.anbehav.2004.12.014 McGrew WC 1992. Chimpanzee Material Culture. Implications for Human Evolution. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511565519 McGrew WC 1983. Animal foods in the diets of wild chimpanzees (Pan troglodytes): Why cross-cultural variation? J Ethol 1:46–61. https://doi.org/10.1007/BF02347830 McLennan MR, Hockings KJ 2014. Wild chimpanzees show group differences in selection of agricultural crops. Sci Rep 4:5956. https://doi.org/10.1038/srep05956 Moscovice LR, Issa MH, Petrzelkova KJ et al. 2007. Fruit availability, chimpanzee diet and grouping patterns on Rubondo Island, Tanzania. Am J Primatol 69:487–502. https://doi.org/10.1002/ajp.20350 Nishida T, Uehara S 1980. Chimpanzees, tools and termites: Another example from Tanzania. Curr Anthropol 21:671–672. Nishida T, Wrangham RW, Goodall J, Uehara S 1983. Local differences in plant-feeding habits of chimpanzees between the Mahale Mountains and Gombe National Park, Tanzania. J Hum Evol 12:467–480. https://doi.org/10.1016/S0047-2484(83)80142-0 O’Malley RC, Power ML 2012. Nutritional composition of actual and potential insect prey for the Kasekela chimpanzees of Gombe National Park, Tanzania. Am J Phys Anthropol 149:493–503. https://doi.org/10.1002/ajpa.22151 O’Malley RC, Power ML 2014. The energetic and nutritional yields from insectivory for Kasekela chimpanzees. J Hum Evol 71:46–58. https://doi.org/10.1016/j.jhevol.2013.09.014 O’Malley RC, Wallauer W, Murray CM, Goodall J 2012. The appearance and spread of ant fishing among the Kasekela chimpanzees of Gombe: Possible case of intercommunity transmission. Curr Anthropol 53:650–663. https://doi.org/10.1086/666943 Pascual-Garrido A 2017. Termite fishing by Mahale chimpanzees: Revisited, decades later. Pan Afr News 24:15–19. Pascual-Garrido A 2018. Scars on plants sourced for termite fishing tools by chimpanzees: Towards an archaeology of the perishable. Am J Primatol 80:e22921. https://doi.org/10.1002/ajp.22921 Sakamaki T, Nakamura M, Nishida T 2007. Evidence of cultural differences in diet between neighboring unit groups of chimpanzees in Mahale Mountains National Park, Tanzania. Pan Afr News 14:3–5. https://doi.org/ dx.doi.org/10.5134/143476 Sanz CM, Deblauwe I, Tagg N, Morgan DB 2014. Insect prey characteristics affecting regional variation in chimpanzee tool use. J Hum Evol 71:28–37. https://doi.org/10.1016/j.jhevol.2013.07.017 Schöning C, Humle T, Möbius Y, McGrew WC 2008. The nature of culture: Technological variation in chimpanzee predation on army ants revisited. J Hum Evol 55:48–59. https://doi.org/10.1016/j.jhevol.2007.12.002 Schuppli C, van Schaik CP 2019. Animal cultures: How we’ve only seen the tip of the iceberg. Evol Hum Sci 1:1–13. https://doi.org/10.1017/ehs.2019.1 Stewart FA, Piel AK 2014. Termite fishing by wild chimpanzees: New data from Ugalla, western Tanzania. Primates 55:35–40. https://doi.org/10.1007/s10329-013-0362-6 Takahata Y 1982. Termite-fishing observed in the M group chimpanzees. Mahale Mountains Chimpanzees Research Project Ecological Report 18:1–3 Uehara S 1982. Seasonal changes in the techniques employed by wild chimpanzees in the Mahale Mountains, Tanzania, to feed on termites (Pseudacanthotermes spiniger). Folia Primatol 37:44–76. https://doi.org/10.1159/000156020 Webster TH, McGrew WC, Marchant LF, Payne CLR, Hunt KD 2014. Selective insectivory at Toro-Semliki, Uganda: Comparative analyses suggest no ‘savanna’ chimpanzee pattern. J Hum Evol 71:20–27. https://doi.org/10.1016/j.jhevol.2014.02.005 Whiten A, Horner V, de Vaal FBM 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437:737–740. https://doi.org/10.1038/nature04047 Wondra EM, van Casteren A, Pascual-Garrido A, Stewart F, Piel AK 2016. A new report of chimpanzee ant-fishing from the Issa Valley, Tanzania. Afr Primates 11:1–18. Received: 4 June 2020 Accepted: 19 June 2020 Back to Contents |