|
<NOTE>
Elementary Technology Correlates with Lifetime Reproductive Success in Wild Chimpanzees, but Why?
Constance Mackworth-Young
& William C. McGrew
Department of Archaeology & Anthropology, University of Cambridge, UK INTRODUCTION In evolutionary ecological terms, if females 'convert' resources into offspring, then accessibility and utilizability of resources must be essential to female reproductive success. Acquiring and processing resources depends on a multitude of factors, from competition (inter- and intraspecific, contest vs. scramble, etc.) to technique (discerning, disarming, extracting, etc. prey). For chimpanzees (Pan troglodytes), much is known about these factors in a general sense: Chimpanzee females compete mostly indirectly by occupying core home ranges within neighbourhoods within group territories (Pusey et al. 1997; Williams et al. 2002; Emery Thompson et al. 2007). Key variables, such as rank, season, sociality, activity budget, diet quality, foraging effort, are interlinked in explaining variation in female fitness (Murray et al. 2006, 2007, 2009). Of these variables, social rank seems likely to be the most important, as manifest in priority of access to resources via scramble competition in core areas of individual female ranging. Most analyses of dietary impact rely on general measures, such as 'diet quality', which may equal fruit availability (Murray et al. 2009), or 'habitat quality', which may equal average plot productivity (Murray et al. 2006). In faunivory, most dietary analyses have been limited to generic meat consumption, with invertebrates sometimes ignored altogether (Tennie et al. 2009), but recent studies confirm the nutritional value of termites (O'Malley & Power 2014). Outcome analyses of reproductive success rely on incomplete and usually contemporaneous records (e.g. fertility, Jones et al. 2010), rather than on lifetime fitness over the whole reproductive lifespan. We know of no previous attempt to relate elementary technology to reproductive success, in any species of primate. Termite fishing is one of the most widespread pattern of technical extractive foraging in chimpanzees, ranging across Africa from Senegal to Tanzania (McGrew et al. 1979; Goodall 1986; Sanz & Morgan 2011). It is the bestknown and longest-studied type of elementary technology in the species, having been first observed at Gombe (Goodall 1963, Lonsdorf 2006). We compare females from the Gombe population in terms of their frequency of using vegetative probes to 'fish' for Macrotermes termites. We relate the females' success in this primary extractive foraging technique in their early lives to their eventual lifetime reproductive success (LRS) achieved, on a variety of measures. We combine data on tool use from a 3-year period in the 1970s with later (2012) data on LRS: age at first birth, offspring survivorship, inter-birth interval, and age at death. We hypothesise positive correlations between frequency of termite fishing and rank and LRS. METHODS Data set We focussed data analysis on all 14 parous females of the Kasekela community in Gombe National Park, for whom from 1972–75 we had observational data. One female, NP, then was excluded, as she had only 6 hr of observation. The remaining 13 females in the community at Gombe were studied by focal-subject sampling (Martin & Bateson 2007) by various researchers over 'follows' of varying duration. We extracted data on bouts of termite fishing (of 5 min duration or longer) from the feeding column of the standard Gombe data-collection instrument, the Travel & Group chart (T&G). Frequency here means total duration of bouts of termite fishing per total feeding time, so the proxy measure for intake of termites was time spent fishing. McGrew & Marchant (1999) showed that time spent fishing and number of soldiers obtained by fishing were highly positively correlated at Gombe. However, two outlier females were excluded from further analyses, for different reasons: GG fished for termites but was sterile (Pusey et al. 1997), so she had zero direct fitness (as per Gilby et al. 2006). FF was fecund but never fished for termites during the study. Despite having more observation hours (175.5) than all but two of the other females, she was not seen to eat termites during the period, although she ate termites at other periods in her life: for example, she ate termites in 1998–2001, but no comparable frequency data were presented (Lonsdorf 2006). We have no explanation for this abstinence over 1972–75; a lengthier, more detailed analysis from the entire Gombe database is needed. Furthermore, FF was the most reproductively successful female in Gombe's history, having given birth first at 13 yr and lived to 46 yr. Seven of her offspring survived to more than 5 yr. As there were no reproductive data for GG and no termite fishing data for FF, we did analyses on the 11 remaining females. Variables Fishing bouts and observation hours were taken from all available T&G records of females as focal targets, totalling 1391 hr and 343 focal follows. This sample is only a fraction of the total T&G data set, which has been collected over decades (see Strier et al. 2010); thus our study should be considered preliminary. Ages were estimated only to the nearest year, as subjects were born before habituation was achieved at Gombe, or were immigrants of unknown origin. Data on (live) births and deaths (to the nearest month) came from the Gombe database. Age at death was taken as last sighting, as most deaths were unobserved. Inter-birth intervals were based on completed birth intervals only, that is, when the next birth followed that of an infant who survived at least 5 yr (to weaning). Criterion for offspring survivorship to 5 yr follows Pusey et al. (1997), but we also added a less stringent criterion of 2 yr (survival through infancy). Scaled ranks for females could not be calculated, given the scarcity of contest competition; instead we assigned categorical ranks (top, high, middle, low), according to published directionality of pant-grunting in dyads (Goodall 1986; Pusey et al. 1997). All statistical tests were done with SPSS Statistics 19. As data were not normally distributed, all tests used were non-parametric. Level of statistical significance (alpha) was set at 0.05, one-tailed. 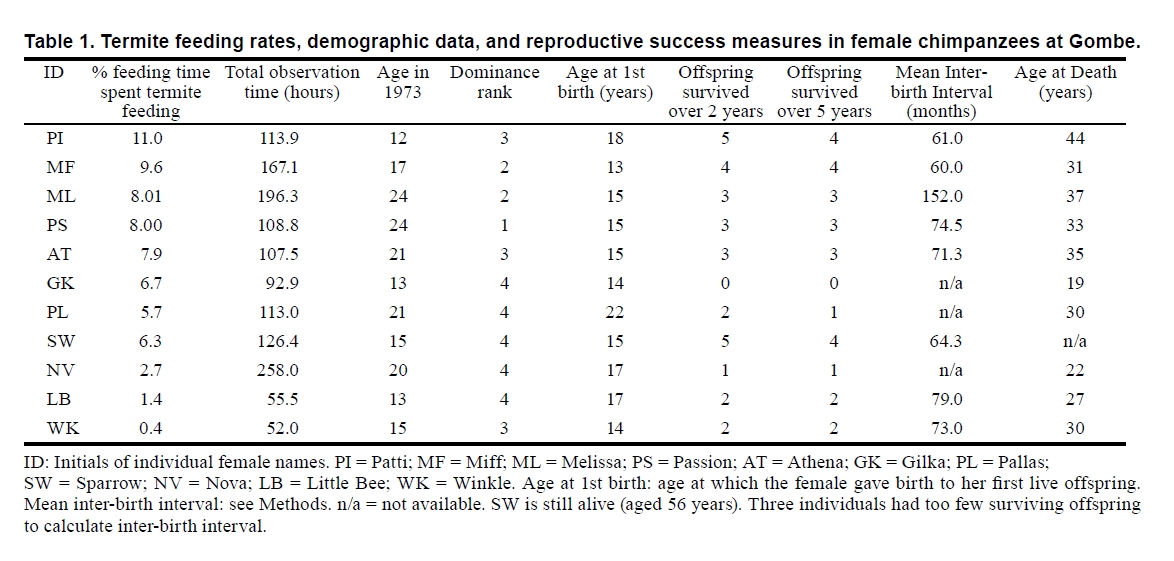
RESULTS Table 1 gives the results of percentage of feeding time spent in termite fishing by individual, as well as their data on five measures of reproductive success. When the termite fishing data were collected, females were 17–18 yr old on average, and most (6 of 11) had had their first live birth at 15–16 yrs of age. The eventual median number of offspring surviving to both 2 yr and 5 yr was 3 (ranges: 0–5, 0–4). Median IBI was 72.2 mo, but three subjects had too few surviving offspring to calculate IBI. Median age of death was 31 yr (range: 19–54+). All of these life history norms resemble other, larger data-sets from Gombe (Goodall 1986). Table 2 gives results of correlations (Spearman's rho) between percentage of observation time spent eating termites and the five dependent variables reflecting LRS. All five variables are in the expected direction of greater LRS. Three of the five measures are individually significantly correlated. Thus, more frequent termite fishers have higher LRS by rearing more surviving offspring and living longer. Social rank also was positively correlated with reproductive success (n = 11, rho = 0.63, p = 0.04, one-tailed). 
DISCUSSION Females who did more termite fishing during their early years of reproductive life had higher reproductive success over their lifetimes. They lived longer and raised more offspring successfully through infancy and through weaning. They tended to have shorter inter-birth-intervals. This may be the first demonstration in a primate species of enhanced reproductive success as a payoff related to elementary extractive technology. Why this correlation occurs is beyond the scope of this pilot study, but it seems most likely to be a matter of individual, differential access to resources (hence the positive correlation with social rank) than a matter of differential skill in termite fishing. It is hard to imagine self-serving technical proficiency in an individual foraging task being linked to dominance status. If access to resources is the key, then a further next step would be to evaluate the abundance, distribution and productivity of Macrotermes mounds in the core ranges of individual females, plus competitive behavior among females. Dominance rank influences use of space by chimpanzee females (Murray et al. 2007) and use of higher quality core areas, at least in terms of fruiting productivity, results in enhanced reproductive success (Emery Thompson et al. 2007). However, termite fishing (a renewable resource) is often done socially, without obvious contest competition (see Figure 1). If social rank is the primary causal variable that determines reproductive fitness, this may be expressed in a variety of ways. Individual variation in efficiency and extent of termite fishing also should be assessed (e.g. McGrew & Marchant 1999; Lonsdorf 2006). Similarly, differential motivation (appetite) for termites, phenotypic quality (Jones et al. 2010), or even general competence at foraging could account for our results. None of these alternative hypotheses can be tested with our data in this preliminary study. However, rank is a constructed attribute, not a behavioral variable. How dominance 'acts', whether generally or specifically, can be elucidated only through focussed studies such as this one. Future studies should make use of comprehensive data-bases that include such variables (e.g. Strier et al. 2010); a more comprehensive study of termite fishing and rank over lifetimes might resolve the correlation/causation quandary. ACKNOWLEDGEMENTS We thank: Caroline Tutin and other researchers at Gombe Stream Research Centre for data; Ian Gilby for additional demographic data from the Gombe database at Duke University, Durham, NC; Anne Pusey and Gen Yamakoshi for helpful comments; Leverhulme Trust for financial support for writing up. REFERENCES Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW 2007. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim Behav 73:501–512. Gilby IC, Eberly LE, Pintea L, Pusey AE 2006. Ecological and social influences on the hunting behaviour of wild chimpanzees, Pan troglodytes schweinfurthii. Anim Behav 72:169–180. Goodall J 1963. Feeding behaviour of wild chimpanzees. Symp Zool Soc London 10:39–48. Goodall J 1986. The Chimpanzees of Gombe: Patterns of Behavior. Belknap, Cambridge, MA. Jones JH, Wilson ML, Murray C, Pusey A 2010. Phenotypic quality influences fertility in Gombe chimpanzees. J Anim Ecol 79:1262–1269. Lonsdorf EV 2006. What is the role of mothers in the acquisition of termite-fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)? Anim Cogn 9:36–46. Martin P, Bateson P 2007. Measuring Behaviour: An Introductory Guide. 3rd edition. Cambridge University Press, Cambridge. McGrew WC, Marchant LF 1999. Laterality of hand use pays off in foraging success for wild chimpanzees. Primates 40:509–513. McGrew WC, Tutin CEG, Baldwin PJ 1979. Chimpanzees, tools, and termites: cross-cultural comparisons of Senegal, Tanzania and Rio Muni. Man 14:185–214. Murray CM, Eberly LE, Pusey AE 2006. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol 17: 1020–1028. Murray CM, Mane SV, Pusey AE 2007. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Anim Behav 74:1795–1804. Murray CM., Lonsdorf EV, Eberly LE, Pusey AE 2009. Reproductive energetics in free-living chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol 20:1211–1216. O'Malley RC, Power ML 2014. The energetic and nutritional yields from insectivory for Kasekela chimpanzees. J Hum Evol 68:46–58. Pusey AE, Williams J, Goodall J 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277:828–831. Sanz CM, Morgan DB 2011. Elemental variation in the termite fishing of wild chimpanzees (Pan troglodytes). Biol Lett 7:634–637. Strier KB, Altmann J, Brockman DK, Bronikowski AM, Cords M, Fedigan LM, Lapp H, Liu X, Morris WF, Pusey AE, Stoinski TS, Alberts SC 2010. The Primate Life History Database: a unique shared ecological data resource. Meth Ecol Evol 1:199–211. Tennie C, Gilby IC, Mundry R 2009. The meat-scrap hypothesis: small quantities of meat may promote cooperative hunting in wild chimpanzees (Pan troglodytes). Behav Ecol Sociobiol 63:421–431. Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J 2002. Female competition and male territorial behaviour influence female chimpanzees' ranging patterns. Anim Behav 63:347–360. Back to Contents |